
News

News
Recently, Prof. Kun Jiang and Prof. Junliang Zhang from the Institute of Fuel Cells and the Interdisciplinary Research Center for Engineering Science, School of Mechanical Engineering have made new research progress in regulating the coordination environment around isolated Platinum single atoms toward selective oxygen reduction reaction. This work, entitled “Manipulating the Oxygen Reduction Reaction Pathway on Pt-Coordinated Motifs”, have been published at Nature Communications. Mr. Jiajun Zhao, a third year postgraduate student, is the leading author. Profs. Jiang and Zhang are the co-corresponding authors.
Electrocatalytic oxygen reduction reaction (ORR) is an important reaction in the process of renewable energy conversion and utilization. Molecular O2 can be reduced via a 4e−-pathway into H2O or via a 2e−-pathway into H2O2. The former serves as the vital reaction in proton exchange membrane fuel cells (PEMFCs) and metal-air batteries to maximize chemical energy conversion efficiency, the latter represents an environmentally benign method for the onsite production of hydrogen peroxide commodity. Therefore, a facile ORR reaction pathway tuning is highly demanded for both fundamental mechanistic investigations and different application scenarios.
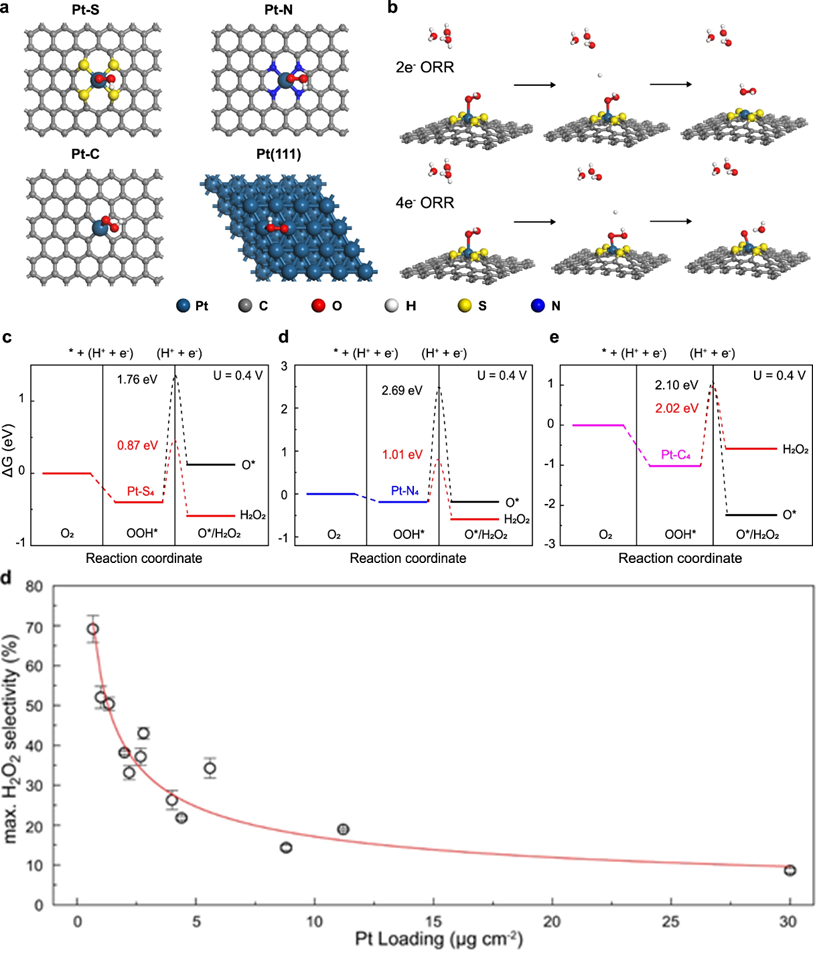
Bulk Pt catalysts are known as the best monometallic materials catalyzing O2-to-H2O conversion, however, controversies on the reduction product selectivity are noted for atomic dispersed Pt catalysts. Here, the authors prepare a series of carbon supported Pt single atom catalyst with varied neighboring dopants and Pt site densities to investigate the local coordination environment effect on branching oxygen reduction pathway. Manipulation of 2e− or 4e− reduction pathways is demonstrated through modification of the Pt coordination environment from Pt-C to Pt-N-C and Pt-S-C, giving rise to a controlled H2O2 selectivity from 23.3% to 81.4% and a turnover frequency ratio of H2O2/H2O from 0.30 to 2.67 at 0.4 V versus reversible hydrogen electrode. Energetic analysis suggests both 2e− and 4e− pathways share a common intermediate of *OOH, Pt-C motif favors its dissociative reduction while Pt-S and Pt-N motifs prefer its direct protonation into H2O2. By taking the Pt-N-C catalyst as a stereotype, the maximum H2O2 selectivity can be manipulated from 70 to 20% with increasing Pt site density, providing hints for regulating the stepwise oxygen reduction in different application scenarios.
Paper Link: Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs

Shanghai Jiao Tong University
Address: 800 Dongchuan Road, Shanghai
200240